Activation Energy of the Iodine Clock Reaction
※ Download: What is the value of the activation energy of the uncatalyzed reaction?
This critical energy is known as the activation energy of the reaction. Now that you know what it takes to get a reaction started, you are ready for the next lesson that describes their actual mechanisms. The same principles will apply to reactions in liquids and solids, but with added complications that we will discuss in a later unit. This the reason that virtually all chemical reactions and all elementary reactions are more rapid at higher temperatures.
Retrieved February 17, 2017. The blue flame sustains itself after the sparks stop because the continued combustion of the flame is now energetically favorable. Such reactions are more properly described as pseudounimolecular. What is the value of the enthalpy change of the uncatalyzed reaction in reverse?

Activation Energy of the Iodine Clock Reaction - Unimolecular processes also begin with a collision The cyclopropane isomerization described above is typical of many decomposition reactions that are found to follow first-order kinetics, implying that the process is unimolecular.

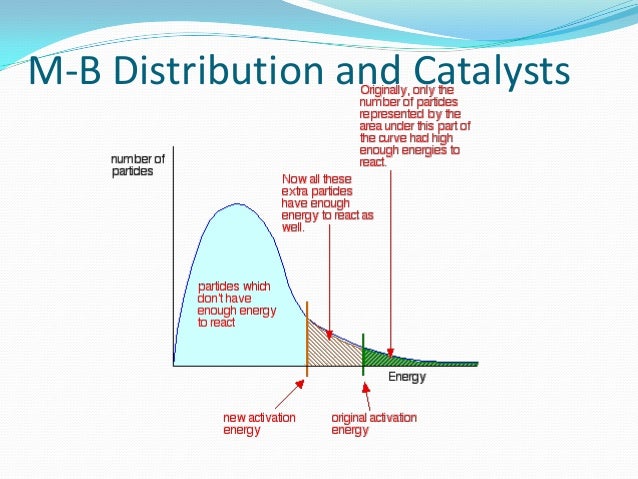
Activation energy is the amount of energy that needs to be supplied in order for a reaction to proceed. This example problem demonstrates how to determine the activation energy of a reaction from reaction rate constants at different temperatures. Activation Energy Problem A second-order reaction was observed. The constant at 3 °C was found to be 8. What is the activation energy of this reaction? Solution Activation energy is the amount of energy required to initiate a. If less energy is available, a chemical reaction is unable to proceed. If you were to make a plot of the energy of the reaction versus the reaction coordinate, the difference between the energy of the reactants and the products would be ΔH, while the excess energy the part of the curve above that of the products would be the activation energy. Keep in mind, while most reaction rates increase with temperature, there are some cases in which rate of reaction decreases with temperature. These reactions have a negative activation energy. So, while you should expect activation energy to be a positive number, be aware it's possible for it to be negative. Who Discovered Activation Energy? In a diagram, activation energy is graphed as the height of an energy barrier between two minimum points of potential energy. The minimum points are the energies of the stable reactants and products. Even exothermic reactions, like burning a candle, require energy input. In the case of combustion, a lit match or extreme heat starts the reaction. From there, the heat evolved from the reaction supplies the energy to make it self-sustaining.
When the bond absorbs energy either from heating or through a collisionit is elevated to a higher quantized vibrational state indicated by the horizontal lines that weakens the bond as its length oscillates between the extended limits corresponding to the curve. Elementary reactions exhibiting these negative activation energies are typically barrierless reactions, in which the reaction proceeding relies on the capture of the molecules in a potential well. This is far more important than memorizing specific examples. The graphs shown here are called potential energy diagrams. Keep in mind, while most reaction rates increase with temperature, there are some cases in which rate of reaction decreases with temperature. This fraction can run from zero to nearly unity, depending on the magnitudes of E aand of the temperature. The minimum points are the energies of the stable reactants and products. Gallery of activation energy plots Activation energy diagrams can describe both exothermic and endothermic reactions:. Part A What is the value of the activation energy of the uncatalyzed reaction. We have arrhenius equation which relate rate constant and activation energy A is pre-exponential factor. For a chemical reaction, or to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. Express your answer as an integer and include the appropriate units.



